The Gut Punch Weekly #17
There's only one story that really matters this week – Eli Lilly Surpasses Novo Nordisk as Lilly's daily oral pill matches Ozempic for weight loss.
Top Stories
1) Eli Lilly's Daily Pill Matches Ozempic for Weight Loss
Eli Lilly's new GLP-1 pill, orforglipron, has shown similar efficacy in reducing blood sugar and aiding weight loss in Type 2 diabetes patients as popular injectable drugs like Ozempic and Mounjaro, according to recent Phase III trial results.
Side effects of orforglipron were reported to be similar to those of injectable GLP-1 drugs, including gastrointestinal issues like diarrhea, indigestion, and nausea.
Lilly's Chief Scientific Officer emphasizes the potential global impact, stating that injections cannot be the solution for the projected billions of people who will have Type 2 diabetes or obesity in coming decades.
The pill offers a potentially transformative alternative to injections due to the ease of oral administration, lower production costs, and wider accessibility.
Eli Lilly plans to seek FDA approval for orforglipron for both obesity and diabetes.
Lilly's shares surged 13% on the announcement.
2) Eli Lilly Surpasses Novo Nordisk in Obesity Drug Market
Eli Lilly has leapfrogged Novo Nordisk in the obesity drug market, thanks to Lilly's superior commercial and clinical portfolios.
BMO has downgraded Novo's shares from "outperform" to "market perform" due to rival Lilly's projected growth and promising cardiometabolic candidate, oral GLP-1 orforglipron.
Recent head-to-head data and reliable supply have contributed to physicians' preferences for Lilly's tirzepatide.
Analysts predicting this trend will continue despite Novo's early lead with semaglutide.
3) Companies Race to Develop GLP-1 Pills
Eli Lilly announced positive phase 3 trial results for orforglipron, a once-daily pill that mimics GLP-1, showing comparable efficacy to injectable GLP-1 drugs in reducing blood sugar and body weight in type 2 diabetes patients.
Small-molecule GLP-1 drugs like orforglipron offer advantages over peptide-based treatments – including lower production costs, easier administration, and improved stability without refrigeration.
Multiple pharmaceutical companies are developing small-molecule GLP-1 drugs, with some exploring innovative approaches targeting different receptor sites to potentially improve efficacy and reduce side effects.
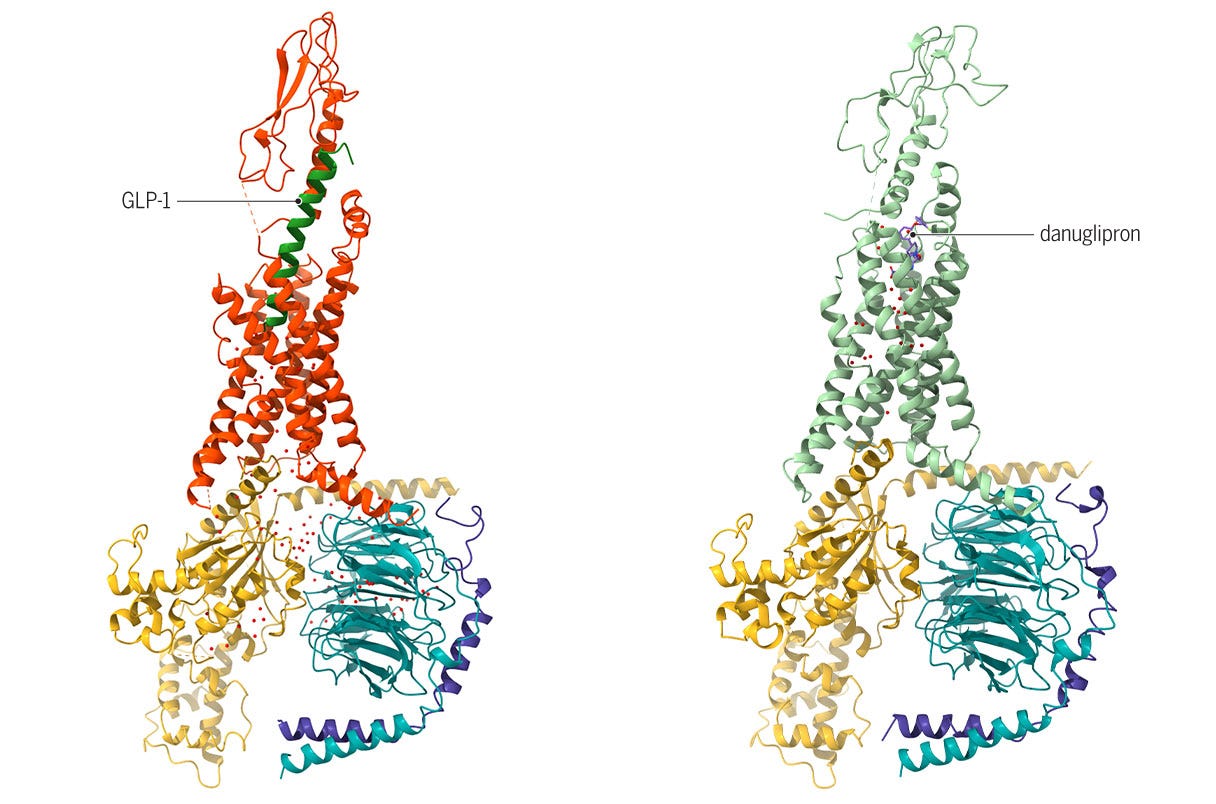
While small-molecule GLP-1 drugs show promise, challenges remain – including potential side effects as evidenced by Pfizer's recent decision to halt development of danuglipron due to liver injury concerns in a trial participant.
(Science)
GLP-1 Industry Intel
Making GLP-1 Pills Proves Challenging for Drug Makers: While oral GLP-1 weight-loss medications could offer greater accessibility than injections, they face significant challenges including gastrointestinal breakdown and strict dosing requirements.
Eli Lilly's Weight Loss Pill Rivals Ozempic, Mounjaro: Eli Lilly's new weight loss pill, Orforglipron, proves as effective as injectable counterparts like Ozempic and Mounjaro in trials.
Novo Nordisk Seeks FDA Approval for Oral Obesity Drug: Novo Nordisk has submitted an FDA application for an oral version of its obesity drug Wegovy, amid increasing competition from Eli Lilly's similar pill orforglipron.
Food & Wellness Industry Intel
Legal Battle Over Compounded GLP-1 Drugs Intensifies: Med spas face legal challenges over compounded GLP-1 drug use amid FDA and pharmaceutical lawsuits.
Food Industry Adapts Products for GLP-1 Users: Food manufacturers are creating new product lines specifically tailored to GLP-1 medication users that focus on high-protein, fiber-rich options to maximize nutritional value in smaller portions.
Americans' Protein Obsession Drives Nutrition Experts Crazy: Food manufacturers are racing to meet consumer demand for protein-enriched products, launching twice as many protein-branded items in 2024 compared to the previous year even as nutrition experts warn against overlooking whole food sources.
Startups Leverage Fermentation for Natural Food Color Alternatives: As the FDA moves to phase out synthetic food dyes, startups like Michroma, Chromologics, and Phytolon are developing fermentation-derived natural food colors that promise superior stability and performance compared to traditional plant-based alternatives.
Frontline Focus
Blue Cross MA Ends Weight Loss Drug Coverage Amid Rising Costs: Blue Cross Blue Shield of Massachusetts will cease coverage of GLP-1 weight loss drugs like Ozempic and Wegovy starting January 2026, citing unsustainable costs that doubled to $300 million last year.
Employers Struggle to Afford Rising Costs of GLP-1 Drugs: Employers face mounting financial challenges as they attempt tp balance GLP-1 drug costs and employee health benefits.
Health Insurers Face Huge Losses From Weight-Loss Drug Costs: Insurers such as Blue Cross are facing enormous financial strain due to the rapid adoption of expensice GLP-1 weight-loss drugs.
FDA Crackdown Threatens Access to Affordable Weight Loss Drugs: The FDA's crackdown on compounded weight-loss drugs threatens to disrupt treatment for many Americans who relied on these cheaper alternatives.
Women Lead in Awareness of Obesity Drugs: A study to be presented at the European Congress on Obesity shows that 80% of UK adults are aware of GLP-1/GIP weight loss drugs, with women showing notably higher awareness and comprehension than men.
ADA Guidelines Prioritize GLP-1 and Early Diabetes Tech: The American Diabetes Association's updated 2025 Guidelines emphasize GLP-1 benefits and med tech use for better diabetes care.
GLP-1 Clinical Insights
Study Finds Limited Sexual Side Effects with GLP-1 Drugs: While GLP-1 receptor agonists showed statistically significant links to male sexual dysfunction in FDA data, the low reporting ratios suggest minimal risk for most patients.
Bremelanotide Enhances Appetite Control, Prevents Weight Rebound: Bremelanotide prevents weight rebound and matches tirzepatide in appetite suppression, showing promise for long-term obesity management.
The Bleeding Edge
GLP-1 Drugs Offer New Hope for Bardet-Biedl Syndrome: A new study published in the Journal of Clinical Investigation suggests that GLP-1 receptor agonists could effectively treat Bardet-Biedl Syndrome, a rare genetic disorder, by improving metabolic function and reducing food intake.