The Gut Punch Weekly #12
Food industry leaders convene for MISTA's Healthy Nutrition Symposium, Obesity drugs need price cuts to be cost-effective, Roche expands obesity pipeline with $1.65B Zealand deal, and more!
Opportunities in a GLP-1 World
The food industry is facing an unprecedented challenge: a new class of drugs is dramatically changing how people eat. GLP-1 agonist medications – like Ozempic, Wegovy, and Mounjaro – have emerged as powerful appetite suppressants by mimicking a gut hormone that regulates hunger. Originally developed to treat diabetes, they are now reshaping what consumers buy, how they snack, and how companies innovate.
It’s one thing for a new diet craze to ask consumers to choose differently; it’s another for a prescription drug to make them feel differently. As appetite literally shuts down and satiety spikes, major food and beverage categories feel the impact.
Analysts warn billions of dollars of consumer spending are up for grabs. KPMG projects consumers may spend $48 billion less on food and drink each year for the next decade due to GLP-1 use. Some of the world’s biggest players – Nestlé, Coca-Cola, Danone, Conagra – are already pivoting to meet the GLP-1 moment.
“We don’t want to be the last guy,” admits Conagra Senior VP Bob Nolan.
So how should the food industry respond? To explore this question, I convened a panel of four experts at MISTA’s 2025 Healthy Nutrition Symposium:
Cate Ward (Semper Organics): Scientist, dietitian, and Stanford University researcher whose work intersects nutrition and microbiome science.
Jenny Zegler (Mintel): Director of global food and drink research at Mintel with 17 years covering food and beverage innovation.
Emilie Fromentin (Givaudan): Head of Explore Health, Functional, and Color at Givaudan, a global leader in flavors, ingredients, and nutritional solutions.
Rocio Martin (Danone): Senior Director of Life Science Innovation at Danone, focused on biotics, proteins, and overall health benefits in food.
Our conversation reveals how GLP-1 medications are reshaping consumer behaviors at a biological level – and how food companies can position themselves to not only survive but thrive in this new world.
Read insights from our conversation in Opportunities in a GLP-1 World.
Top Stories
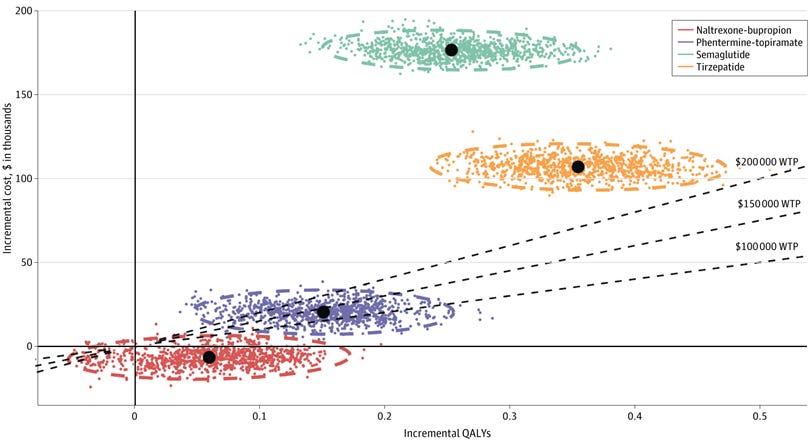
1) Obesity Drugs Need Price Cuts To Be Cost-Effective
A cost-effectiveness analysis of antiobesity medications found that tirzepatide and semaglutide offered the greatest health benefits, averting over 45,000 and 32,000 obesity cases per 100,000 individuals respectively.
However, while tirzepatide and semaglutide provided the largest quality-adjusted life year gains, they are not cost-effective at their current high prices, which translate to $197,023/QALY and $467,676/QALY respectively.
These drugs require substantial price decreases to reach a $100,000/QALY threshold – 30.5% for tirzepatide and 81.9% for semaglutide.
Policy solutions are needed to improve affordability and access to effective antiobesity medications, including facilitating generic drug entry, expanding Medicare coverage, and exploring alternative weight maintenance approaches.
2) Roche Expands Obesity Pipeline with $1.65B Zealand Deal
Roche has agreed to pay Zealand Pharma up to $5.3 billion for the rights to petrelintide, an experimental amylin-targeting obesity drug currently in Phase 2b trials.
The agreement allows Roche to explore combinations of petrelintide with its existing obesity drugs, including the GLP-1/GIP agonist CT-388 acquired from Carmot Therapeutics.
Roche's deal with Zealand Pharma for petrelintide is yet another sign of the growing interest in amylin analogs for obesity treatment, following AbbVie's recent $350 million licensing agreement with Gubra and despite mixed results from Novo Nordisk's amylin-GLP-1 combination therapy, CagriSema.
The partnership leverages Roche's manufacturing capabilities, addressing potential supply challenges that have affected competitors like Novo Nordisk and Eli Lilly.
3) Weight Loss Drug Race Expands Beyond Fat Loss
With multiple GLP-1 agonists demonstrating 15-25% weight loss in clinical trials, companies are now focusing on differentiating their products through additional health benefits and indications.
This shift is driven by the need to justify coverage to reluctant payers and provide value beyond weight loss alone.
Eli Lilly's Zepbound and Novo Nordisk's Wegovy have expanded their approvals to include sleep apnea and cardiovascular risk reduction, respectively, while other companies are pursuing indications like MASH, kidney disease, and Alzheimer's.
The development of oral GLP-1 formulations is expected to improve patient persistence and accessibility, with Eli Lilly's orforglipron Phase III trial readout anticipated to be a major industry event in 2025.
Companies like Structure Therapeutics and Terns Pharmaceuticals are also advancing oral GLP-1 candidates.
(Biospace)
4) Opko and Entera Partner on Novel Oral Obesity Drug
Opko Health and Entera Bio have formalized a partnership to develop the first orally administered dual agonist GLP-1/glucagon peptide.
The partnership will combine Opko's OPK-88006 with Entera's N-Tab oral delivery technology.
As part of the agreement, Opko will purchase 3.6 million shares of Entera at $2.17 each to help fund development costs through the Phase 1 trial.
The oral GLP-1/glucagon agonist aims to treat obesity, metabolic disorders, and fibrotic diseases as a once-daily tablet, potentially offering an alternative to injectable therapies like Novo Nordisk's Wegovy and Eli Lilly's Zepbound.
The companies plan to seek FDA permission to begin human trials later this year.
5) NotCo Launches Food-Based Alternative to Weight Loss Drugs
NotCo, a Chilean food tech startup, has developed a GLP Booster powder that stimulates natural GLP-1 production in the body, offering a food-based alternative to weight-loss drugs like Ozempic.
The GLP Booster, set to launch this year, will be available as a powdered blend for consumers to add to foods and as an ingredient for food manufacturers to incorporate into packaged products.
NotCo's CEO emphasizes that the product is not intended to replace GLP-1 drugs but to complement them and offer benefits to those who cannot access such medications.
The product aims to help users feel satiated and eat less, while potentially mitigating side effects and weight rebound associated with GLP-1 medications.
NotCo's AI-driven approach to developing its GLP Booster aligns with two major growing trends among food companies: 1) creating food-based alternatives and supplements to capitalize on the GLP-1 trend, and 2) leaning into AI to speed up product development and launch timelines.
GLP-1 Industry Intel
CDMO Recipharm Expands Syringe Capacity for GLP-1s: Contract manufacturer Recipharm posted strong 2024 results with €827 million in revenue and expanded manufacturing to capitalize on future GLP-1 drug manufacturing opportunities.
New Long-Acting GLP-1 Implant Begins Human Testing: Vivani Medical has successfully initiated and fully enrolled its first-in-human clinical trial of NPM-115, a twice-yearly GLP-1 implant designed to improve medication adherence in obesity treatment.
Altimmune Tests Obesity Drug for Alcohol Disorders: Altimmune plans to expand testing of its GLP-1/glucagon agonist pemvidutide into Phase II trials for both alcohol use disorder and alcohol-related liver disease, joining competitors in exploring GLP-1 treatments for alcohol-related conditions.
Biolexis's AI Platform Discovers Novel GLP-1 Drug Mechanism: Biolexis Therapeutics has developed BLX-7006, a novel oral small-molecule GLP-1 receptor agonist with a unique allosteric binding mechanism.
Food & Wellness Industry Intel
Natural Weight Loss Supplements Fall Short of Ozempic: Despite claims about 'natural Ozempic' alternatives like berberine and green tea, experts say there's no evidence that supplements can match the significant weight loss effects of prescription GLP-1 medications.
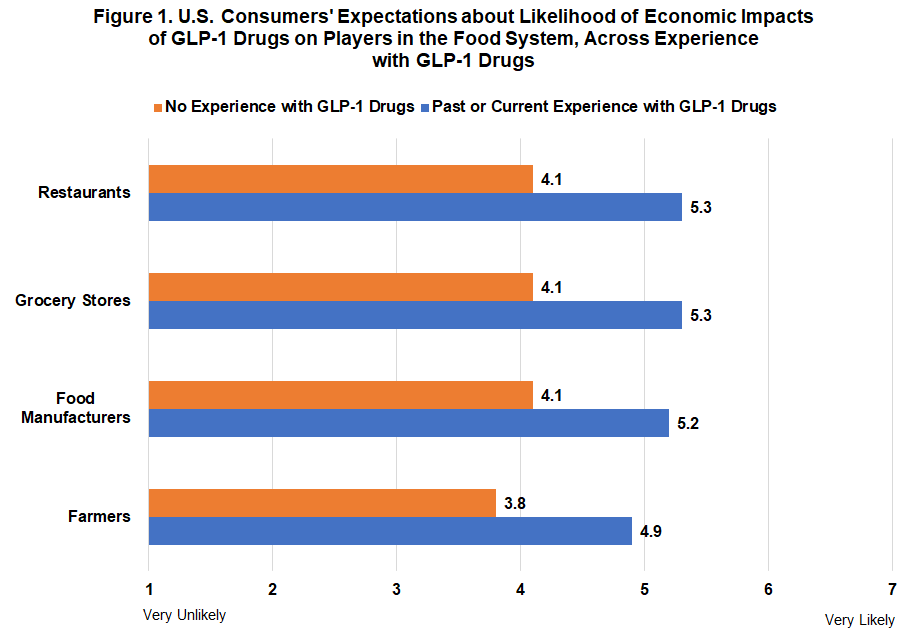
GLP-1 Users Predict Significant Restaurant and Grocery Impact: According to a Gardner survey, consumers with GLP-1 medication experience strongly believe these drugs will transform food purchasing behaviors and impact profitability across the food supply chain, from farmers to restaurants.
Yerba Mate Shows Promise for Metabolic Health: A comprehensive review of yerba mate research reveals its potential for managing metabolic disorders through multiple pathways including gut microbiota modulation, appetite regulation, and improved mitochondrial efficiency.
LifeVantage GLP-1 System Expands Globally After Strong Sales: LifeVantage's MindBody System, designed to naturally activate the body's GLP-1 production, is expanding internationally starting with a phased launch in Japan.
PatchMD Launches Nutrient Patches for GLP-1 Users: PatchMD has launched a new collection of transdermal patches specifically designed to support GLP-1 medication users by addressing common side effects and nutrient deficiencies through skin-based delivery.
Frontline Focus
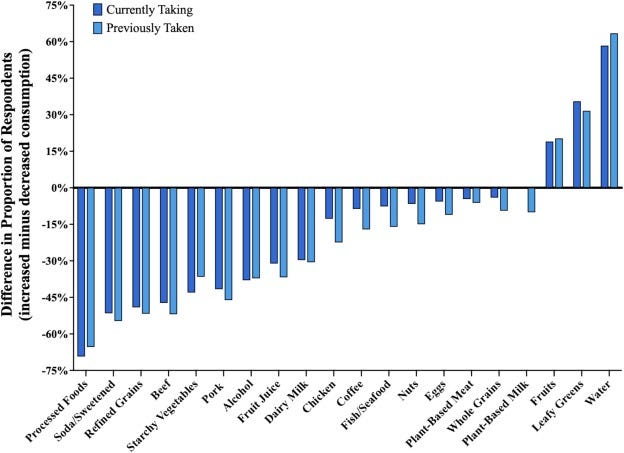
GLP-1 Users Cut Calories, Shift Food Preferences: Survey data from nearly 2,000 respondents indicates GLP-1 medications significantly reduce caloric intake and alter food consumption patterns, particularly decreasing intake of high-calorie processed foods, sodas, and refined grains – while increasing intake of fruits and vegetables.
Patients Fear Loss of Cheaper GLP-1 Drug Options: The FDA's crackdown on compounded GLP-1 medications following the end of drug shortages is forcing patients to choose between expensive branded options or potentially losing access to their treatments altogether.
FBI Warns of Dangerous Fake Weight Loss Drugs: The FBI warns that counterfeit versions of popular weight loss drugs Ozempic and Wegovy are being sold at suspiciously low prices and may contain dangerous substances instead of legitimate semaglutide.
Expert Challenges RFK Jr's Views on Ozempic: A clinician challenges RFK Jr.'s views on weight-loss drugs, emphasizing that medications like Ozempic are necessary tools for patients whose bodies resist traditional weight-loss methods.
GLP-1 Clinical Insights
Semaglutide With Lifestyle Changes Shows Strongest Weight Results: Research involving nearly 10,000 participants demonstrates that GLP-1RAs combined with lifestyle changes resulted in greater weight loss (-7.49kg) compared to GLP-1RAs alone, with semaglutide showing the best results.
Study Shows Semaglutide Pregnancy Risks Similar to Insulin: Research examining semaglutide use during pregnancy revealed comparable risks to insulin-exposed pregnancies for complications like preterm birth and neonatal issues, but showed higher risks compared to unexposed pregnancies.
Hormone Therapy May Boost GLP-1 Success for Postmenopausal Women: According to a Mayo Clinic study, postmenopausal women using hormone therapy lost significantly more weight with semaglutide than non-users, achieving 16% total body weight loss compared to 12% after 12 months.
Hair Loss More Common in Women Taking Wegovy: A new study found that people taking semaglutide had a 50% higher risk of hair loss compared to those on older weight-loss medications, with women experiencing twice the risk of men.
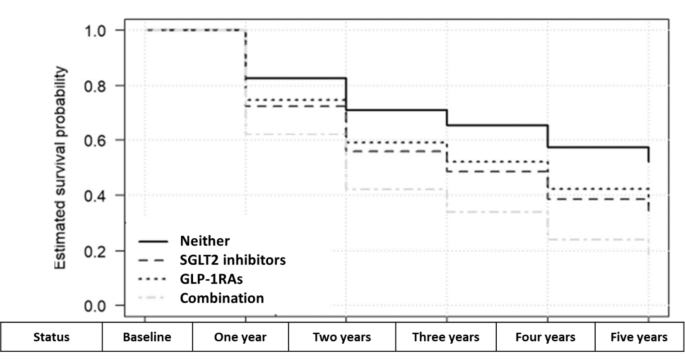
Diabetes Drugs SGLT2 and GLP-1RA Increase Fall Risk: New research indicates SGLT2 inhibitors pose a significant fall risk for type 2 diabetes patients, and the combination with GLP-1RAs shows nearly triple the fall risk compared to using neither medication.
GLP-1 Weight Loss Before Hip Surgery Raises Mortality Concerns: New research indicates that rapid weight loss using GLP-1 medications before hip replacement surgery may pose unexpected mortality risks despite reducing some surgical complications.
GLP-1 Drugs Show Promise for Inflammatory Bowel Disease: Research shows that GLP-1 receptor agonists are well-tolerated in IBD patients with no increase in disease flares and significant weight loss achieved in most patients.
The Bleeding Edge
New Drug Retatrutide Cuts Cancer Growth By 14x: The weight-loss drug retatrutide demonstrated powerful anti-cancer effects in both obesity-related and non-obesity-related cancers through metabolic improvements and immune system modulation.
New Semaglutide Microspheres Show Improved Drug Release Control: Scientists created innovative semaglutide microspheres that overcome traditional drug delivery limitations by utilizing low molecular weight PLGA and Tween 20 to achieve consistent, controlled release without lag phases.
Study Shows PYY and Exendin-4 Alter Taste Perception: A study demonstrates that oral application of PYY and exendin-4 peptides can enhance taste-related behaviors and perception in mice, particularly increasing their responsiveness to fatty substances.
Metformin and Semaglutide Show Promise for Parkinson's: A new study found that co-treatment with metformin and semaglutide provides enhanced neuroprotection against Parkinson's disease in diabetic rats by improving antioxidant systems and reducing inflammation.
Study Shows Konjac Fiber Controls Appetite Through Gut-Brain Connection: Scientists found that konjac fiber's unique expandable properties help control appetite by affecting digestive processes as well as hormone levels, and neural pathways in the stomach-intestine-brain axis.